Mother's day is a much bigger deal than Father's day. Why? Well, there's just something extra special about mom (sorry Dad!). So, today's post is about an under-appreciated group of moms (you guessed it), Arizona black rattlesnakes!

Human moms - you think you have it tough? Rattlesnake maternal duties may only last a couple weeks, but during that time they may have to protect their kids from extreme temperatures, a suite of predators, annoying (and deadly?) squirrels, and clumsy humans with cameras... By the time they give birth, mother rattlesnakes likely haven't eaten in weeks or even months, but they wait another couple weeks to give full attention to their newborns. So here's to you rattlesnake mommies!
We'll start with the most famous of all, Cap Mama, who showed us what a typical day is like for a new rattlesnake family:
For an explanation of the behaviors seen in that video, check out this post.

What a beautiful family she has!
Sigma may have been one of our smaller mothers, but what she lacked in size she made up for in bravery:
Check out the full story of Sigma's squirrel battles here.
We've been lucky enough to see Woody and Alice with two different litters.

Alice's family, 2010

Alice's family, 2012
Woody's family, 2012. You can watch more timelapse videos of Woody's family here and here.
Every mom needs a day off. So the lucky (or smart?) rattlesnakes that nest in groups help each other out with maternal duties. If one is still pregnant, and thus needs to be on the surface basking, she attend to the newborns while the new mother stays in cover for a well-deserved rest. Priscilla was the first rattlesnake we observed exhibiting this baby-sitting behavior.
 You can read more about Priscilla and House here.
You can read more about Priscilla and House here.
Male rattlesnakes occasionally help out in this way too. Although we've never observed any active care or protective behavior from males, just the presence of a large rattlesnake may be enough to deter some predators.
Still image of the group:

Green Male (adult male) is the large black rattlesnake at the top of the image and the mother (Devil Tail) is the smaller, brown adult (mostly her tail and rattle are visible).
A handful of newborns follow Roger (adult male) out of the nest entrance to a preferred basking spot.
Sometimes the youngest (smallest) mom gets stuck with the surface duties of caring for the newborns. Eve was the smallest of the pair of snakes that nested at this site; we saw her often on the surface with way too many babies to have all been her own. The older (larger) female was rarely seen on the surface with the newborns.

This is the first Mother's day in years that we haven't spent at dens with our rattlesnake mothers-to-be. But, as of last week, two (Persephone and Luna) of our three Muleshoe rattlesnakes are still near their dens. While this is atypical rattlesnake behavior in general, it is characteristic of pregnant Arizona black rattlesnakes. So maybe we'll have a couple more names to add to this list next year!
Sunday, May 12, 2013
Monday, November 26, 2012
 |
| From the website "How can you cuddle without arms?" |
I was contacted a while ago by a student in the Animal Behavior class at Reed College. For this class, pairs of students design a website on an animal behavior of their choice and this student was working on social snake behavior.
Check out their excellent website, "How can you cuddle without arms?".
What a fun and useful assignment! I am totally impressed with this class, but of course you'd expect great things from a class with this on their homepage:
Wednesday, October 17, 2012
When someone says social behavior, rattlesnakes are probably not the first animal that comes to mind. However, lizards exhibit a variety of complex, social interactions: stable family group-living, social and genetic monogamy, and cooperation (Chapple 2003, Davis et al. 2011, McAlpin et al. 2011).
Recently, Dr. Rulon Clark and colleagues documented cryptic sociality in rattlesnakes: pregnant female and juvenile timber rattlesnakes tend to aggregate with their kin. You can download and read the full article here.
We analyzed association patterns to describe social behavior of individual rattlesnakes. Specifically, we investigated two questions:
Gregariousness is an individual's propensity to be social.
Arizona black rattlesnakes (Crotalus cerberus) possess many traits linked to sociality: long lifespan, late maturation, and they care for their offspring. Important to this study, Arizona black rattlesnakes aggregate (group) at sites they use year after year (philopatric), are mainly diurnal, and phenotypically variable (individuals looked different).
We used remote timelapse cameras (Wingscapes Timelapse PlantCam and TimelapseCam 8.0) to record behavior of rattlesnakes within aggregations. We set up 1–3 cameras at each basking site, and they took photographs every 30 s from ~8am–6pm. We stitched these photographs into videos, which were lumped into day-long sampling periods.
Here we present results from one basking site (more to come from two additional sites!)
We primarily identified individual rattlesnakes using natural aberrancies in their dorsal patterns (see Zona's story for more information). When we captured rattlesnakes away from aggregation sites, we painted their rattles, which both assisted in identification and validated the pattern method.
This is an example of what our data look like, for one site on one day. Rattlesnakes observed within a body length were defined as in association; thus for rattlesnakes to be considered in association, we had to observe them together at least once during a particular day.
The next step was to take those observations of associated rattlesnakes and estimate the amount of time they spent together.
An association index (AI) estimates the amount of time each pair of rattlesnakes spent together. Because we were not always able to identify every individual in a group, we used the half-weight AI, which is less biased in these situations (Whitehead 2008, Cairns and Schwager 1987). The half-weight AI places more emphasis on the occasions when both animals are observed together than when one is observed without the other.
So now we have an estimate of the proportion of time each pair of rattlesnakes at this site spends together. We used the program SOCPROG to calculate AI's and test our research questions. Because AI's are not independent, SOCPROG implements a version of the permutation test developed by Manly (Bejder et al. 1998). This permutation test shuffled group membership within sampling periods (days) to produce a data set that represented what our data would have looked like if snakes were associating randomly. We then compared our observed data to the random data to see if rattlesnakes associated non-randomly.
Our measure of gregariousness was typical group size, or the mean group size experienced by an individual rattlesnake (Jarman 1974). We looked for differences in variation in typical group size (standard deviation = sd) between observed and randomly permutated data generated in SOCPROG.
Gregariousness varied among individual rattlesnakes; some rattlesnakes preferred to be alone or in small groups, while others preferred large groups.
Again, we are looking at variation in AI's between observed and random data. High variation in observed data, relative to random data, means that some pairs of rattlesnakes often associate (high AI) while others avoid each other (low AI).
Rattlesnakes are choosy about whom they do and do not associate with. At this site, observed variation in AI was significantly greater than random data overall and among female-female and female-juvenile pairs.
So YES - rattlesnakes are social. As mentioned above, we are currently analyzing data from two other basking sites and have a few more ideas to follow up this study:
We plan to use social network analysis to describe this population of rattlesnakes,
investigate how kinship affects association patterns,
and because we restricted our analyses to associations between Arizona black rattlesnakes as they emerged from their dens, we're interested in association patterns during the active season and in other rattlesnake species (left: black-tailed rattlesnakes in combat, Crotalus molossus; right: western diamond-backed rattlesnakes 'stacking', Crotalus atrox).
Our study describes social behavior in a 'non-social' species and may provide information on the evolution of sociality. But more importantly, our findings have the potential to impact conservation of an entire group of misunderstood and often maligned organisms. Humans have an innate fascination with snakes (Burghardt et al. 2009), but unfortunately, this keen emotional response is often appropriated by fear. Pernicious myths and popular media that have long portrayed snakes as malicious villains instill and nurture fear of snakes, which has led to widespread persecution and obstruction to conservation efforts (Seigel and Mullin 2009). In contrast to how snakes are usually seen in the media, recent research on rattlesnakes reveal behaviors that appeal to the general public: parental care (Greene et al. 2002, Amarello et al. 2011), cooperation, and social interactions (Clark et al. 2012, this study). By revealing the social nature of snakes, we are starting to change the public's perception of snakes from 'cold-blooded killer' to social creatures with complex family lives and make a positive impact on their conservation.
References and further reading
Amarello, M., J. J. Smith and J. Slone. 2011. Family values: Maternal care in rattlesnakes is more than mere attendance. Available from Nature Precedings.
Bejder, L., D. Fletcher and S. Brager. 1998. A method for testing association patterns of social animals. Animal behaviour 56:719-725.
Burghardt, G., J. Murphy, D. Chiszar and M. Hutchins. 2009. Combating ophiophobia: origins, treatment, education and conservation tools. Pp. 262-280 in S. J. Mullin and R. A. Seigel, eds. Snakes: ecology and conservation. Cornell University Press, Ithaca, NY, USA.
Cairns, S. J. and S. J. Schwager. 1987. A comparison of association indices. Animal Behaviour 35:1454-1469.
Chapple, D. G. 2003. Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetological Monographs 17:145-180.
Clark, R. W., W. S. Brown, R. Stechert and H. W. Greene. 2012. Cryptic sociality in rattlesnakes (Crotalus horridus) detected by kinship analysis. Biology Letters, doi: 10.1098/rsbl.2011.1217.
Davis, A. R., A. Corl, Y. Surget-Groba and B. Sinervo. 2011. Convergent evolution of kin-based sociality in a lizard. Proceedings of the Royal Society B: Biological Sciences 278:1507-1514.
Greene, H. W., P. May, D. L. Hardy Sr., J. M. Sciturro and T. Farrell. 2002. Parental behavior by vipers. Pp. 179-205 in G. W. Schuett, M. Hoggren, M. E. Douglas, and H. W. Greene, eds. Biology of the vipers. Eagle Mountain Publishing, Eagle Mountain, Utah. USA.
Jarman, P. J. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48:215-267.
McAlpin, S., P. Duckett and A. Stow. 2011. Lizards cooperatively tunnel to construct a long-term home for family members. PLoS ONE 6.
Seigel, R. A. and S. J. Mullin. 2009. Snake conservation, present and future. Pp. 281-290 in S. J. Mullin and R. A. Seigel, eds. Snakes: ecology and conservation. Cornell University Press, Ithaca, NY, USA.
Whitehead, H. 2008. Analyzing Animal Societies. University of Chicago, Chicago.
Thursday, July 19, 2012
Ever since we saw Roger Repp's talk at the Tucson Herpetological Society, Burrow Buddies — or Not?, we've been fascinated by different reptile species sharing shelter sites. Multiple species often share the same overwintering site; we shared this fun example here back in April:
At one of our new dens at Muleshoe Ranch, we have seen western diamond-backed rattlesnakes, spiny lizards, Gila monsters, coral snakes, patch-nosed snakes, earless lizards,
Arizona black rattlesnakes,
and Sonoran whipsnakes (cruising by while an Arizona black rattlesnake sits at the right side of the den opening).
We've also seen western diamond-backed rattlesnakes and Gila monsters sharing den sites elsewhere:
And, lizards that would be prey for rattlesnakes during the active season also share den sites with their potential predators:
But what about during the active season?
There are probably chance encounters like this:
 A black-tailed rattlesnake cruises by a resting Arizona black rattlesnake (Boyett).
A black-tailed rattlesnake cruises by a resting Arizona black rattlesnake (Boyett).Jaydin, a black-tailed rattlesnake we are radio-tracking at Muleshoe Ranch, spent a couple weeks shedding his skin in a particular rockpile earlier this summer. Last week we happened to be walking by that rockpile and even though we knew Jaydin was long gone, we looked underneath to see if anyone else was using it:
Sure enough, there was a juvenile western diamond-backed rattlesnake resting under the rock. For whatever reasons, these shelter sites serve the needs of different individuals of different species. In this case the visits by the black-tailed and western diamond-backed rattlesnakes were weeks apart, but what if they needed to use the rock at the same time?
A few days ago, a friend took us out to visit some rattlesnake nests. We stopped at a site that was being used this year by Sunny, a pregnant ridge-nosed rattlesnake, but found this little guy instead:
Where was Sunny? Did she move to a new nest site? Was she resting behind the rock rattlesnake? In more than 20 years of studying ridge-nosed and rock rattlesnakes, our friend has never seen them intermingle, despite the fact that these species are often found in the same habitat. After an unsuccessful check of other sites Sunny has used, we left to visit some other rattlesnake nests. We returned to Sunny's nest a while later and were greeted with this surprise:
Um, wow! What is going on here? Is this just coincidence or could they be interacting in a mutually beneficial way?
Through careful observation and using time-lapse cameras, we are seeing more examples of different species sharing sites. This is the first time we have heard of or seen two different snake species coiled together like this. If you have, we would love to hear about it in the comments section below - please share!
Thursday, February 23, 2012
Drs. Rulon W. Clark, William S. Brown, Randy Stechert, and Harry W. Greene [1] found cryptic sociality in timber rattlesnakes (Crotalus horridus). Timber rattlesnakes use communal winter dens and pregnant females aggregate together at rookeries to gestate their young. Clark and colleagues collected DNA samples from rattlesnakes to examine relatedness within these aggregations. While all individuals from the same den were not related, aggregations of juveniles of the same age group and pregnant females that shared rookeries were related.

An adult male with several female and juvenile Arizona black rattlesnakes basking just outside their den.
Timber and Arizona black rattlesnakes are similar in many aspects of their behavior. Arizona blacks also den communally, although this behavior is not restricted to the northern part of their range as it appears to be in timber rattlesnakes [1,2]. Some female Arizona blacks use rookeries at or near their dens, although many nest alone.
So, are aggregations of Arizona black rattlesnakes related? Or like timber rattlesnakes, perhaps only aggregations of pregnant females and juveniles are related. Or maybe aggregations of Arizona black rattlesnakes are just random groups of unrelated individuals. This is one our research topics, which you can read more about here. Hopefully we'll be able to answer the above questions over the next couple years.
- Clark, R.W., W.S. Brown, R. Stechert, and H.W. Greene. Cryptic sociality in rattlesnakes (Crotalus horridus) detected by kinship analysis. Biology Letters rsbl20111217; published ahead of print February 22, 2012. doi:10.1098/rsbl.2011.1217
- Brown, W.S. 1993. Biology, status, and management of the timber rattlesnake (Crotalus horridus): a guide for conservation. Herpetological Circular 22.
Thursday, October 20, 2011
Priscilla, Lois, and Arli spent their pregnancy together at a rookery from May through August, 2010. Arli moved away to a private nest shortly before giving birth.
 Priscilla & House, 1 September 2010
Priscilla & House, 1 September 2010
On 30 August 2010 we observed Priscilla (pregnant adult female) discouraging House (neonate / newborn) from potential exposure to a human predator. Because Priscilla was pregnant at this time, House had a different mother. To our knowledge, this is the first observation of helping - where an animal cares for another's offspring - in a snake. Perhaps this is why some female rattlesnakes aggregate during gestation and remain together after giving birth.
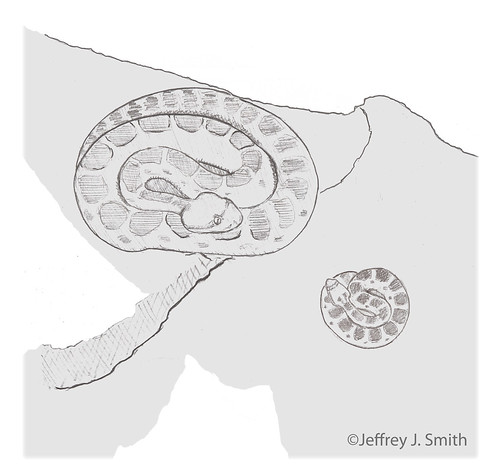 15:27 Priscilla (adult female) and House (neonate) are at rest in a shaded rock shelter.
15:27 Priscilla (adult female) and House (neonate) are at rest in a shaded rock shelter.
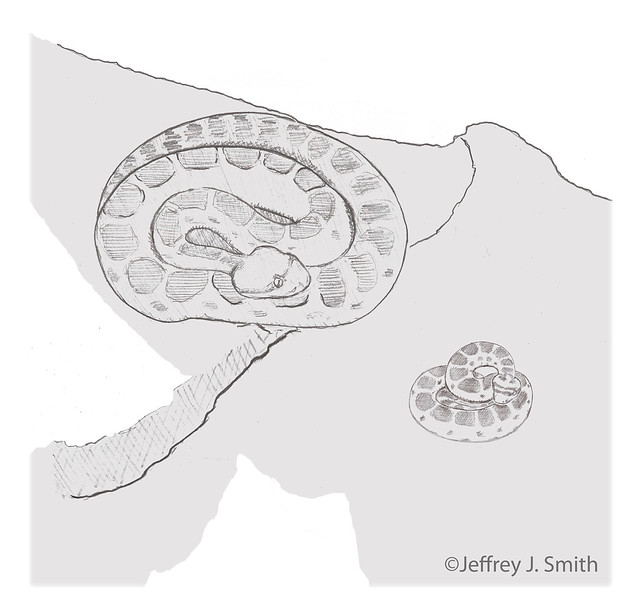 15:28 House moves restlessly in cover and then begins to move toward open ground.
15:28 House moves restlessly in cover and then begins to move toward open ground.
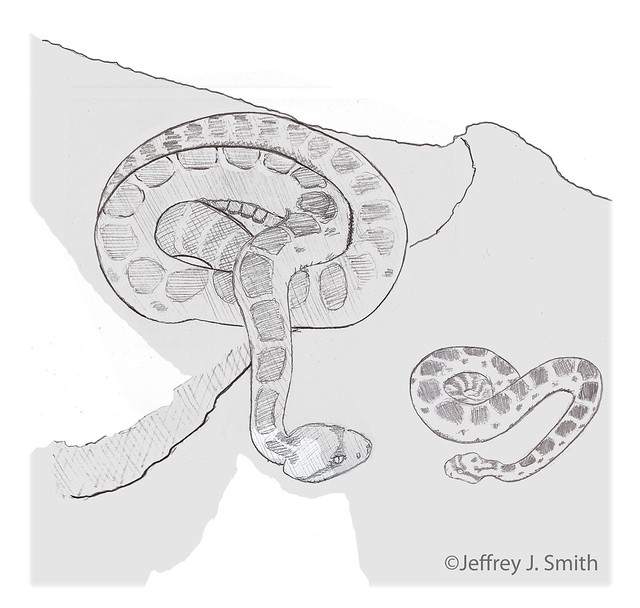 15:29 Priscilla swiftly confronts House before he wanders away from cover; her posture is unusually rigid.
15:29 Priscilla swiftly confronts House before he wanders away from cover; her posture is unusually rigid.
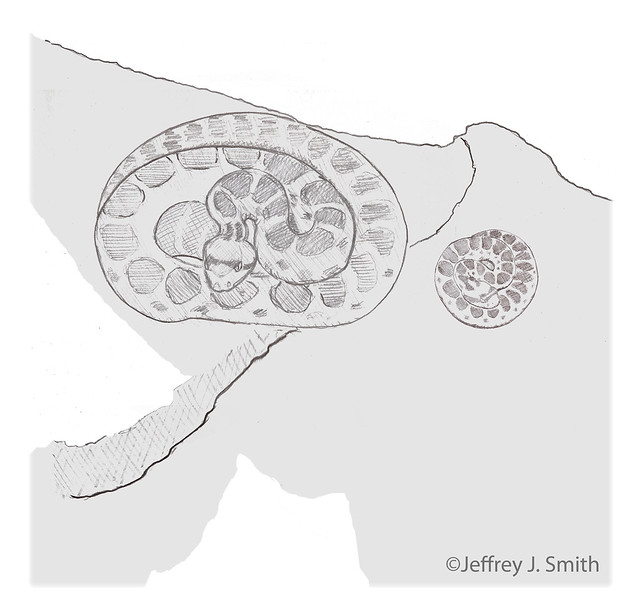 15:31 House stops, turns around, and coils in cover. Priscilla's head returns to her coils.
15:31 House stops, turns around, and coils in cover. Priscilla's head returns to her coils.
Thursday, March 31, 2011
Halfway between


7 March 2011
8 March 2011
18 March 2011














































